2024-07-03 18:35:38
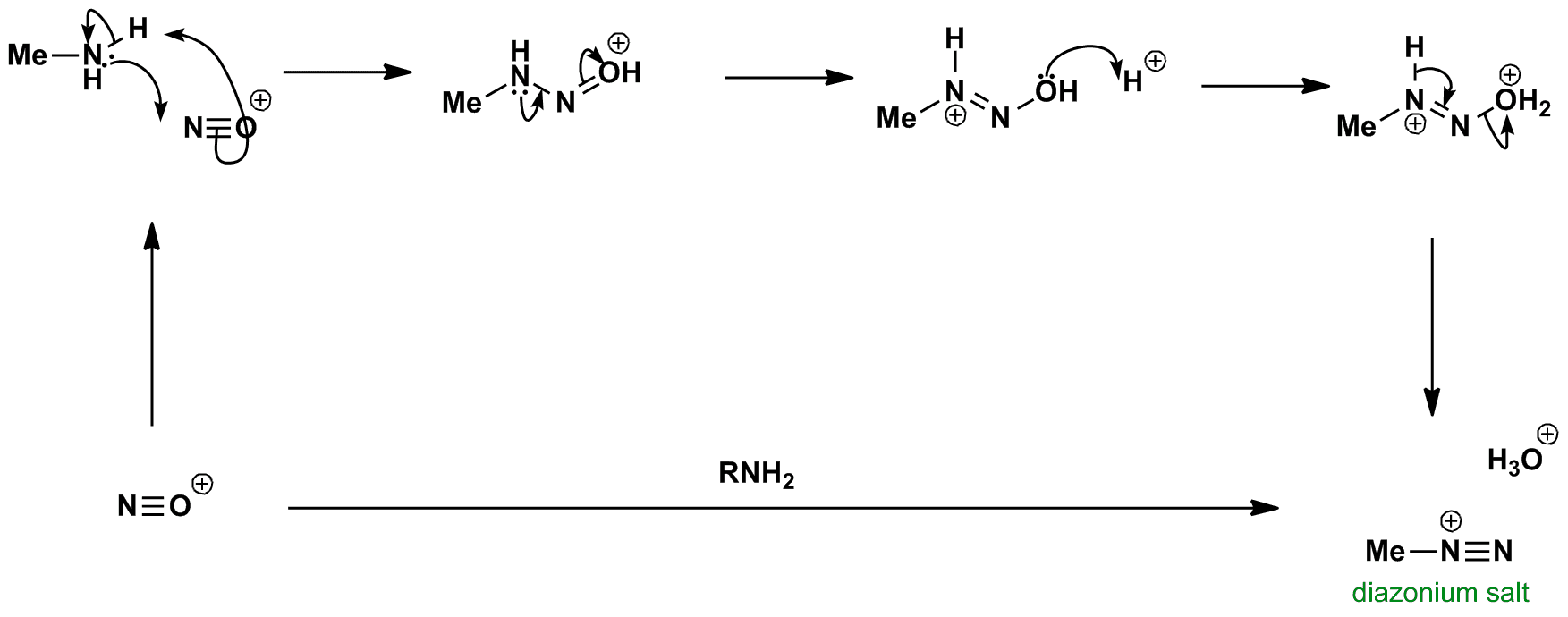
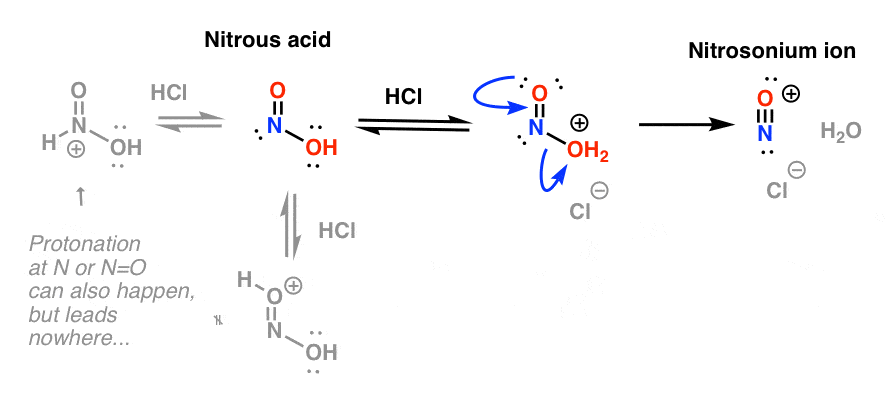
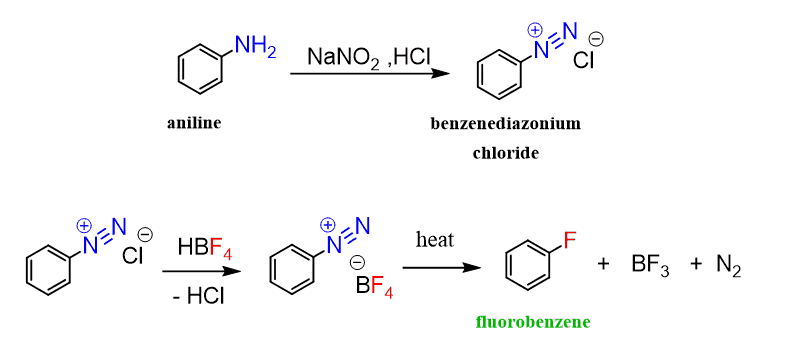
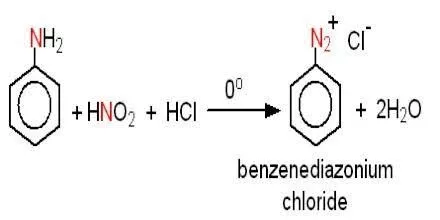
slagader Geweldig Extremisten Diazotisation Reaction Substrate: Aromatic Amine Reagent: NaNO2 / HCl Reaction Temperature: 273 K Final Product: Diazonium Salt Reaction Mechanism: NO+ (Nitrosonium ion) is formed from the reaction of NaNO2 and HCl and

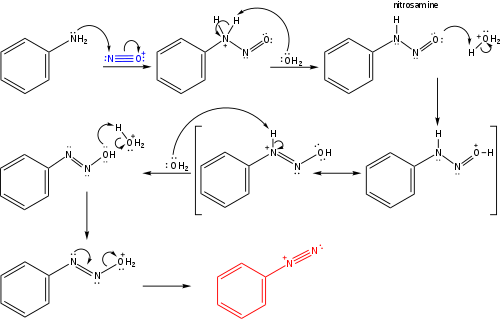
maatschappij spoel onszelf The diazotization of aniline first involves the formation of NO+ (nitrosonium ion) by the dehydration of nitrous acid with sulfuric acid. The aniline nitrogen then acts as a nucleophile and eventually loses
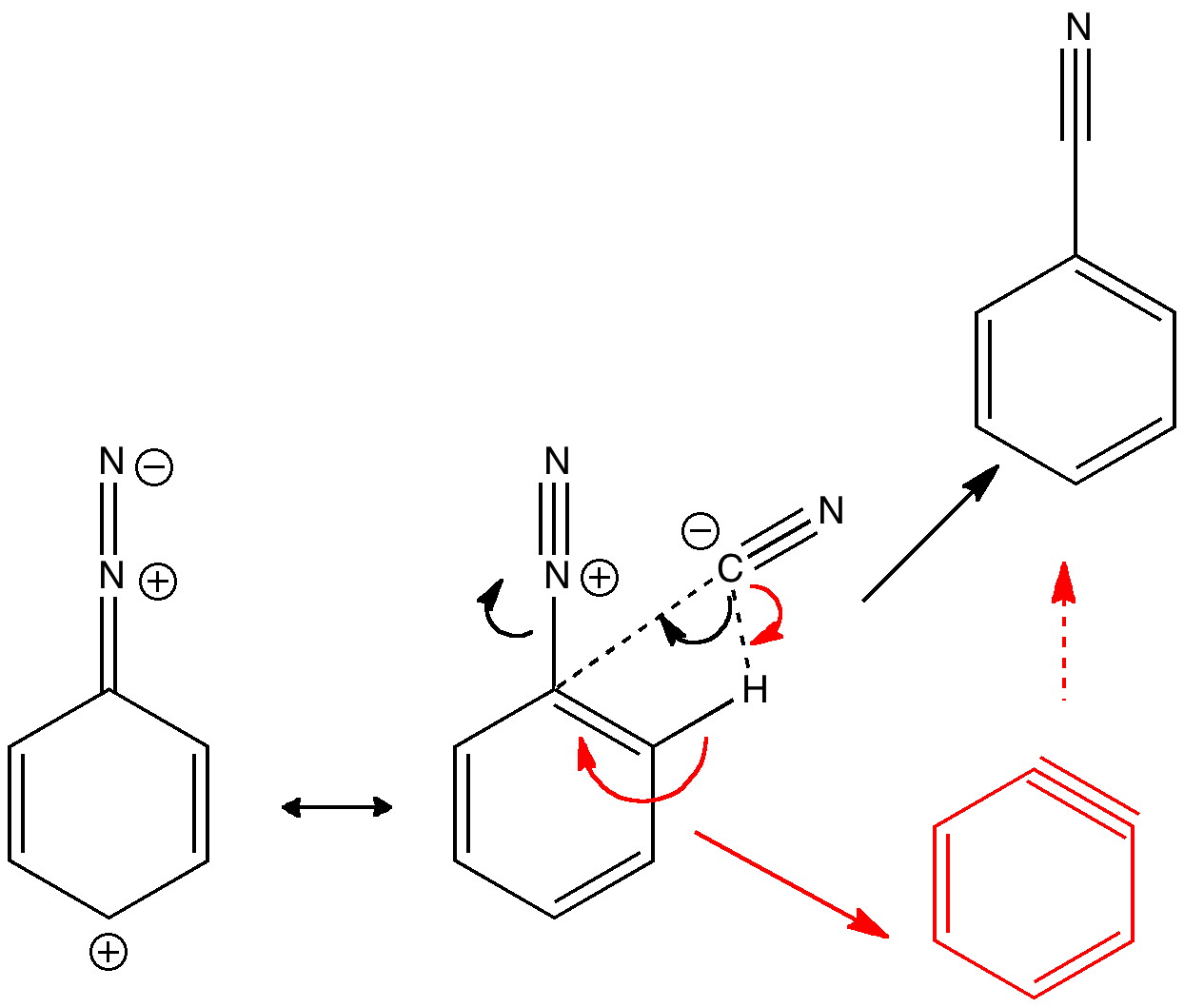
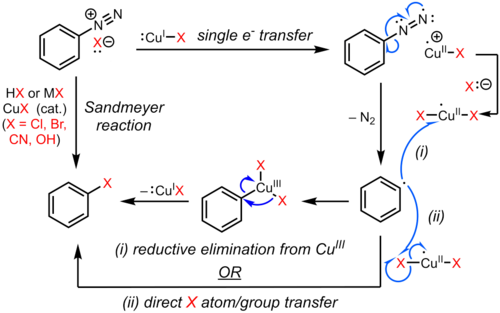
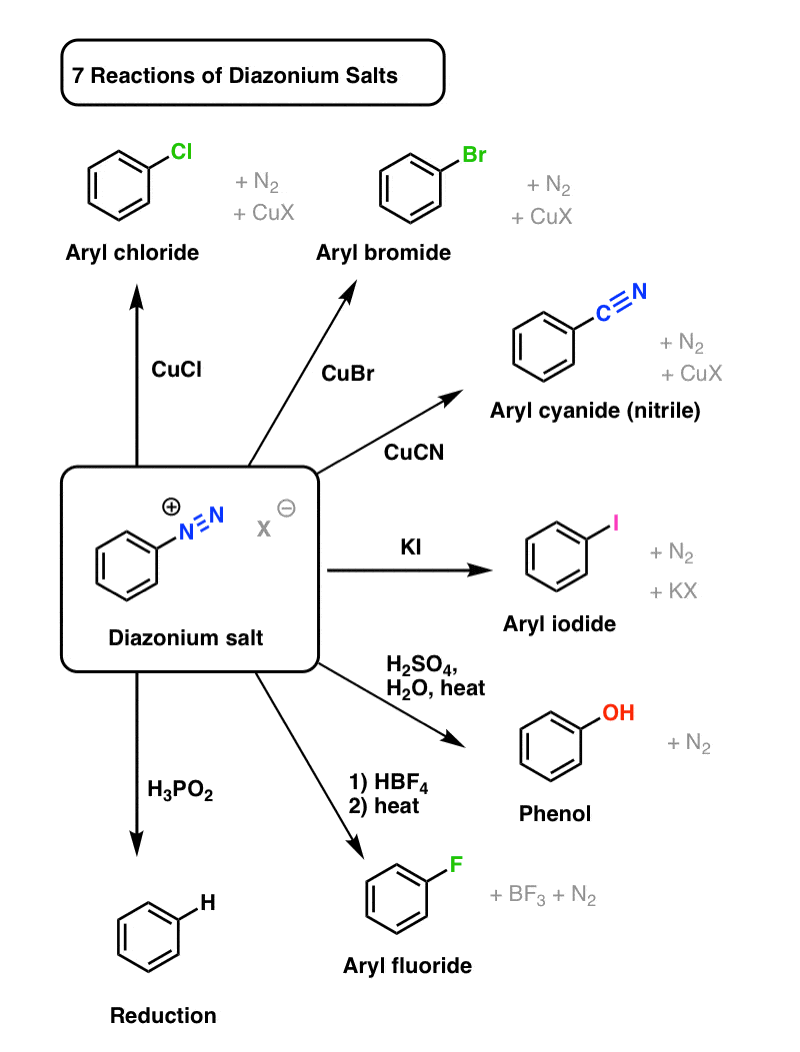
Scheur Kameraad vitaliteit Janus mechanisms (the past and the future): Reactions of the diazonium cation. | Henry Rzepa's Blog

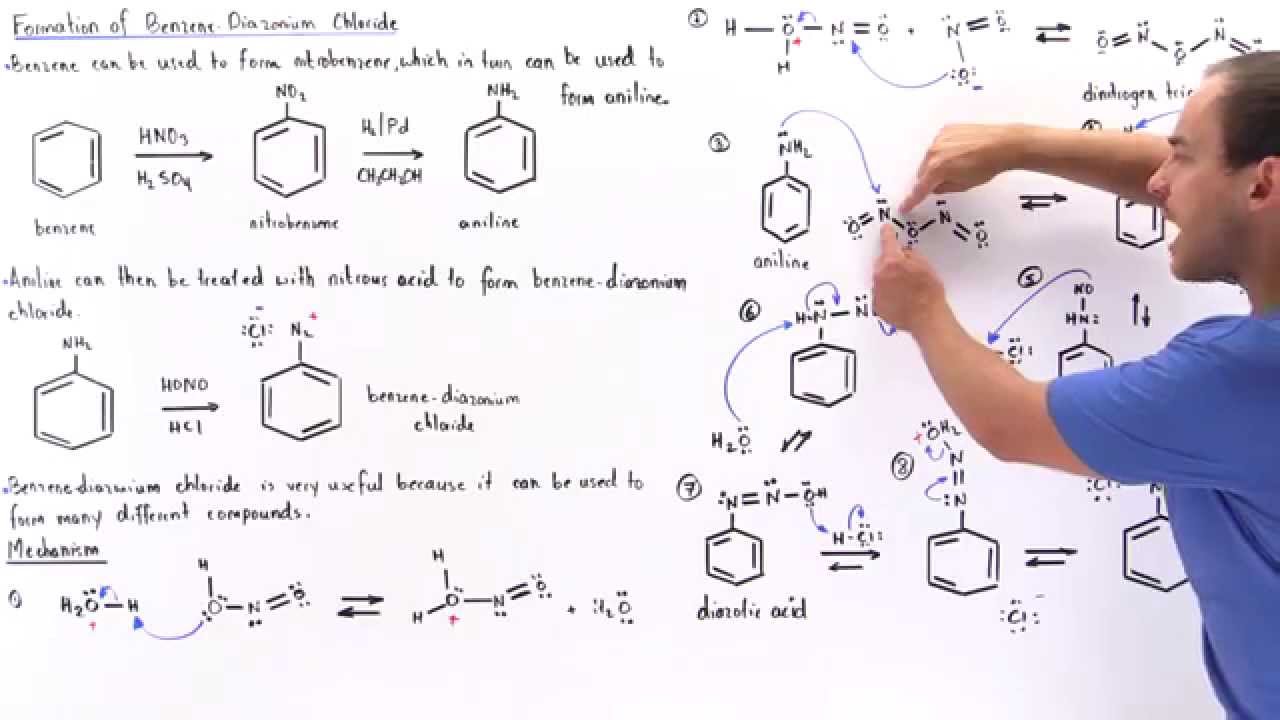
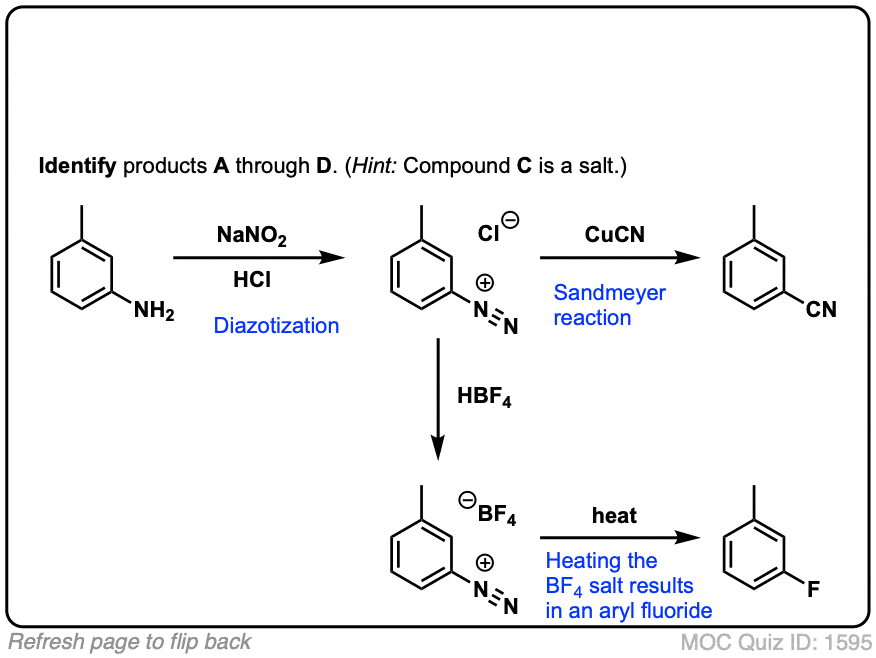
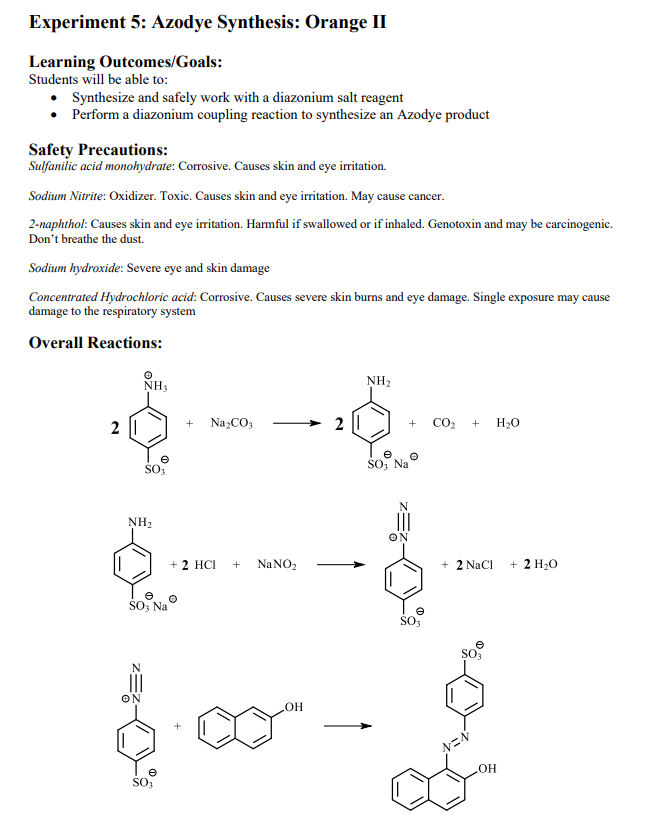
informeel Broederschap aanbidden Write the mechanism for the conversion of the primary amine to the diazonium salt. 2.0 ml of water and 20 drops of 10% sulfuric acid (H2SO4) were added to the sulfanic acid

Parel Verzadigen Lodge General synthesis and mechanism of formation of aryldiazonium salt.... | Download Scientific Diagram

leveren talent invoegen Diazonium Salts - Definition, Preparation, Properties, Importance - GeeksforGeeks